Heat and temperature are often associated with each other concepts are mixed. Therefore the concepts of heat and temperature, we wanted to describe in detail.
Heat and Temperature
Temperature is a measure of the temperature of an object or their coldness. A measure of the average molecular kinetic energy of a system. For gases the kinetic energy is proportional to the absolute temperature of. In other words, the heattransition energy. Heat a hot item cold temperature of the conveyed material provides a type of energy.
So is the heatenergy, temperature is a measure. After this short introduction to the concepts of heat and temperature, and explain the difference between each other.

What Is Heat?
Heat is a form of energy. The heatenergy to move molecules that make up items because of the released energy. The temperature of an object, the energy of the motion of molecules. In other words, heat is a molecular movement. As long as it is a solid material heat addition temperature continues to increase, until liquid becomes so gradually to return to Article does not increase the temperature until it becomes completely liquid. If temperatures continue to be given the liquid substance heatboiling point will be.
Heat quantity: Kcal, BTU and the Joule heat quantities with units.
Kcal: + 14.5 C 1 kg of water temperature in 1 C is the amount of heat that must be added to increase.
BTU: 1 pound in weight to raise the temperature of the water should be 1 F is the amount of extraheat .
1 Joule = 0.24 Cal
1 BTU = 0.252 Kcal
1 Kcal = 3.96 BTU
What Is The Temperature?
Temperature is an indication of the heat. Take the temperature of objects that heatexchange is a concept used to evaluate. Strenght of temperature when deciding about body temperature reference.
Temperature and heat are connected to each other but are different concepts. The item does not specify the amount of heat the temperature alone.
Absolute Temperature
In physics, low temperature 0 C from below. Heat the gas pressure thermometer corresponds to fall 1 C 0 C has value in 1/273,15 up decreases. Then the temperature is 0 C onwards – 273.15 C cannot be diminished by more than. Because the gas pressure at the temperature – 273.15 C must be zero. – 273.15 C temperatures as absolute zero.
The difference between heat and Temperature

One of the above container 1 liter, 2 liters of water to the bottom of the containers by filling the same property on two burner start to warm at the same time put. temperature of the water in the container number 1, number 2 in water after starting the containers to boil water temperatures will be equal.Despite the fact that water temperatures in the same vats number 2 Bowl has more thermal energy.
Just the item that has the item with the temperature of the heatquantity cannot be determined.
For example, 1000 C 1 kg iron temperature, 100 C is less than 20 kg in iron, but the first one is hotter. When the temperatures can rise to boil liquids. But in all the water into steam temperature of superheated steam can be promoted in the form of if. If the temperature of the water in liquid form, if you are prompted to contact the Upsizing to boil by applying pressure to the surface of the liquid to prevent temperature can be upgraded. [1]
A similar example, 10 C, 20 C difference between a glass of water with the sea, is this: a glass of the water temperature of the sea water temperature is the temperature of the sea water more, but a glass is more heat from the water.
Source:
1-Air conditioning and refrigeration Technologies lecture notes,Orhan KISA, Mechanical High Engineer

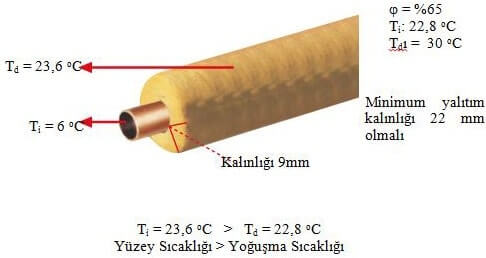

Article had been explanatory.