Coal is composed of non – homogeneous, compact, predominantly lignocellulosic plant fragments, stratified, mostly brown, with a small amount of H – O – S and N elements, but in inorganic (clay, silt, z elements) and solid fossil organic masses that can be burned, with black tones. Coals are used for various purposes (such as coke making, chemical production) as well as fuel raw materials.
In the case of coals, in conditions of marshes, if appropriate conditions are met (such as the presence of humid and warm climates, the presence of sufficient organic matter, the presence of muddy water at pH 4-5, downward sedimentation along with the material development, Some chemical reactions result in the physical and chemical changes of the resulting organic material, which eventually breaks down, disintegrates, becomes a gel with marshy water.
The environments in which the marshes bring the coal to the fountain;
– Deltas (medium of thickest coal veins),
– Lakes (lake bushes, suitable marshy areas where thick coal seams occur),
– lagoons (bringing fine coal-veins of the sea effect)
– River overflow plains (form fine coal veins).

Coalification (“Coalification”)
Often, plant materials or plant parts accumulate in suitable marshy environments, precipitate and are buried underground with geological functions. Underground, these organic masses, after being buried, are affected by the pressure conditions created by the burial prior, and subsequently by the thermal conditions of the environment. The result of this influence is physical and chemical changes in the organic matter. These organic materials, which are known as peat and are known as ancestors of coal, have darker colors over time and have a more rigid structure. The result of the effect of temperature and pressure conditions on these masses is that water and water vapor, carbon dioxide (CO2), oxygen (O2) and most advanced hydrogen (H2) (anthracite phase) escape from this medium, respectively. Of course, in this process the ideal conditions and the thermal conditions of the environment must dominate and increase over a long period (thousands of years). the ground temperature increases by 10 C every 30 meters. Undoubtedly the temperature increase is ideal and normal conditions. Outside of these conditions (volcanic activity, fault movements, in the presence of radioactive elements), the temperature of the earth rises remarkably and normally much more. This organic material, which is called “peat” but is not considered as coal , is transformed into “lignite”, then “bituminous coal”, then “coal”, “anthracite” and finally “graphite”. This progressive maturation process is called “Coalification” and at every level it is called “Rank of coal” (“Rank”).
Coals undoubtedly contain clay, silt, sand and inorganic (mineral) substances in various proportions. These inorganic substances in the coal directly affect the quality of the carp in the negative direction. The quality of a car may have different meanings depending on the area of use. For example; the best quality coal in coke manufacture, the best coal that can be swollen, porous, and resistant, non-oxidized coal. Coking coal as fuel raw materials does not make any sense, the most sought-after feature is that it has very thermal properties. The most sought-after feature of coal liquefaction is the excess of volatile matter. It comes. But on the whole inorganic material is not a desirable component.
Classification of Coals (“ASTM”)
Coals are subjected to many different classifications. European coal is classified as “hard coal (quality coal over lignite)”, “brown coal”. The most commonly used classification is undoubtedly the ASTM (1983), which is in the chart below, and the volatile substance is a classification based on calorie value. In order to be able to identify an inorganic substance well, these classification are sometimes insufficient and organic petrographic studies are also taken into consideration.However, the carbonization grades of the coals are most accurately reflected by their reflection values. In addition to these values, it is also important to know some trace elements (such as Arsenic, Cadmium) which are polluting elements in terms of environment.
Physical and Chemical Properties of Coals
Coals are crisp, crumbly, combustible, and contain organic matter except for inorganic substances. The chemical properties of coal are very common among the people.
Physical Properties of Coals
If you look roughly at the physical properties of coal; Their densities range from 1.1 to 2.2 gr / cm³ despite the increase in inorganic matter and moisture content.
The porosities range from 3% (anthracite) to 25% depending on the degree of carbonization.Hardnesses (Vickers) range from 30 (lignite) to 120 (anthracite) kg / mm².
Coal Reflection Properties
The values of Rmax, Rmean, Rrandom and Rmin are measured in coals. Reflectance values give us the real carbonization ratings of a basin coal, presenting directly the diagenetic properties (thermal values they are subjected to) they had in the past.
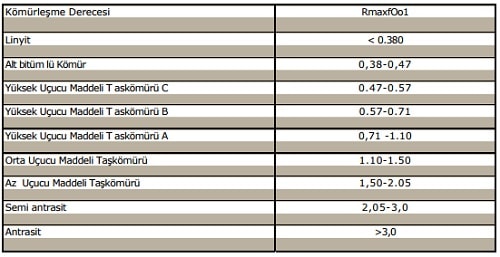
Table 2.% Rmax Values of Coals and Carbonation Grades Ward. 1984 and Stach. 1982).

Table 3. Measured% Reflection (Rmax) values of some coals, Paleo-temperature values and Correspondence Grades Carbonation Grades (Boggs 1987).
The Rmax values of the coals directly give rise to the highest values of temperature, from the past to the present, from day to day. The information gained from this helps us to understand what kind of pressure the media is under and what kind of properties it has.
Vitrinite reflectance is used in petroleum and natural gas searches. Any trees on sedimentary rocks that form petroleum-bearing formations on oil exploration. The measurements we make on the piece give us very great help to find out what kind of hydrocarbons (heavy liquid, liquid, gas, or nothing) can be found or not.
The reflection can vary depending on the deepness of the under-ground nature of the valleys. It is natural that higher coal values are shown due to the influence of more “coarse” coals on the “geothermal gradient” (“Hilt Law”). Other factors that increase the reflection value of coal include mountain formation, volcanic activity, active fault movements, radioactive minerals etc. d.
Fluorescence Properties of Coals
Coals are organic materials and some organic substances have fluorescence properties because they show fluorescence properties. The fluorescence properties of coals reveal exact contrasts with their reflection properties. On the contrary, the fluorescence properties of peat and coal with low reflectance values are high values. As the degree of carbonization increases, the fluorescence properties of liptinite and vitrinite decrease in parallel and disappear at high carbonization levels.
Liptinites are macroscale, which, as mentioned before, are the highest fluorescence properties because they are the latest to participate in the charring event. Apart from some semifusinites, inertinite group macroscale generally show no fluorescence properties. The fluorescence light itself is also examined on the polished specimen and the measurements are made accordingly.Higher fluorescence values appear in lighter colors (light green, etc.), while those with lower fluorescence values appear in darker colors (such as red, dark brown). It is much easier to distinguish lecithinites from their detailed properties and inorganic materials (hair, etc.) with this method.
Coals have many similar physical properties that they exhibit like
Chemical Properties of Coals
Caloric (thermal), short and elemental analyzes reveal the qualities and properties of coal. Trace element analysis can also be done in coal.
Short (“Proximate”) Analysis of Coals
Moisture, Volatile, Ash and Fixed (“FIXED”) Carbon analyzes. These analyzes can reveal their qualities together with petrographic analysis of a cow.
Element (“Ultimate”) Analysis of Coals
Determination analysis of “C”, “S”, “O”, “N”, “H” elements in coal. It can be done for very specific and detailed inquiries.
Coal analysis can be done in some private universities and institutions such as TUBITAK, iron and steel factories, some coal enterprises (TTK and some TKI regional directorates), Hacettepe and ODTÜ “in MTA General Directorate of Mineral Analysis and Technology.
Coal Components
Macroscopic Components of Coals
When we take away a piece of coal, we see that they have different surfaces and looks. Some coals are crumbling in cubic divisions. Plants are visible in vegetation, while some coals have thick, shiny and matte banding.
A piece of coal has a stratification plane at the top and always in a horizontal position, as if it were parallel to the ground surface, if a curl was not broken or displaced.
On the sides of the coals there are planes called “Clit” (perpendicular to the stratification planes and perpendicular to each other). The front or “face” clit is smooth while the “butt” clit is rougher.The side clits become parallel to the fold axes of the rocks of the environment. The clits are formed by influencing factors of the mountain formation of the environment, that is, they are of orogenic origin. The clit angles are at 90 ° celsius at the level of hard coal, and at lower coal levels (lower bituminous coal and lignite) this angle is smaller or different. The number of clits in anthracite is more than two (three), which together provide the conchoidal division.
Especially at the level of coal , coals show banded appearance. When the coals are examined by hand, their banded, matted, handy components are called “lithotypes”. When the lithotypes finish, they take the “ain”. There are four lithotypes different from each other. These are Vitren (“Showcase”), Klaren (“Clarain”), Düren (“Durain”) and Füzen (“Fusain”). The characteristics of the lithotypes are shown in Table 4 in detail. Table 4 also contains features related to coal clay and clayey coal in relation to each other.
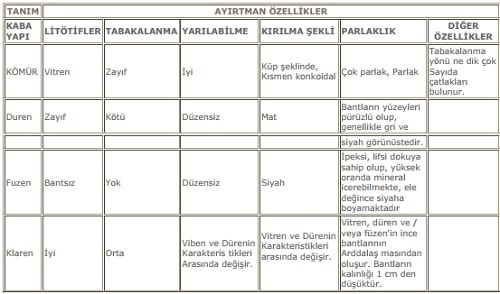
Table 4. Lithoties and properties of bituminous coals.
Microscopic Components of Coals
Coal specimens are investigated in brightly lit microscopes by making polishing briquettes. The examination of the coal in this way has led to the observation of all the detailed properties of the samples.
maceral on
For the similarity of minerals, which are the smallest components in rocks, the smallest units in organic materials are also called maseral.
Masters and minerals that bring the coal to the fountain do not spread well along a coal stratum.On the contrary, if they do not have a control gold, they are especially formed and deposited in the environment due to the biological, chemical and geological functions of the environment.Different conditions allow different properties of organic materials (or maseralin) and minerals to be found in the environment. Undoubtedly, these organic materials (maserals), which are formed under different conditions and whose origins may be different, can have different physical and chemical properties.
Maserals are grouped because of different morphological structures and different physical and chemical properties in coal. They get the word “init” at the end. There are three main maseral groups. These main groups are Vitrinite (huminite in lignite and lower bituminous coals), Liptinite (former Ekzinite) and Inertinite.
microlithotypes on
Even in small micro areas (about 50 microns), sometimes the masters are not alone and are found with other masters. One or more coexisting maseral communities can be considered as “microlithotip”. In rocks, concepts such as rock units (granite, gneiss) correspond to lithotypes in coal and minerals in macels in coal. But the microlithotype concept is an intermediate concept that can be studied with microscopes, which has a similar size between the lithotypes and the masses, and which is similar to the rocks, without a defined definition. These microlithotites specified in the classifications shown in Table 6 are used more extensively for special purposes (in determining the environments in which the coal is formed). Microlithotisms take the “it” tag instead of the “init” tag in the maseral at the end.

Table 6. Microlithotites and Their Components (Stach, 1982).
Source: Dr. Selami TOPRAK, General Directorate of MTA

