In 1985 it was found that free chlorine compounds in the atmosphere damaged the ozone layer. It was also found that there were large ozone holes on Antarctica and the Arctic. Accordingly, a timetable for deactivation of CFC-based refrigerants by the Montreal Protocol and the 1990 London Meeting was established in 1988. The use and production of CFCs has been banned completely in the G-7 countries since 1995. HCFC-based fluids, such as transition-period gases R22 and R502, will be completely deactivated by 2030.Again, in 1992, the Clean Air Act entered into force in the United States and CFC-based refrigerants were forbidden to vent atmospheres.
As the CFCs rise on the ozone layer, they are exposed to UV radiation. Free chlorine (Cl) atoms are released in the atmosphere. When a chlorine atom is collided with an ozone compound (O3), one of the oxygen atoms (O) is bonded in the form of chlormonoxide (ClO), the other two oxygen atoms separated are an oxygen molecule. When the ClO molecule contacts a free oxygen atom, the oxygen atoms combine to form an oxygen molecule (O2), and the chlorine atom (Cl) remains free to disrupt another ozone molecule. Each free chlorine atom can destroy 100 000 ozone molecules before returning by natural means such as troposphere rain.

The disintegration of ozone

Growth of ozone hole depending on years
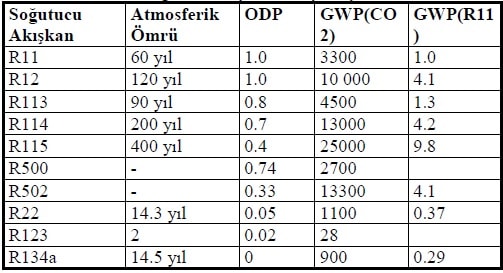
The effect of CFCs on ozone is called Ozone Drilling Factor (ODP), and R11 gas is referred to for this factor. New HFC type fluid R123 gas for R11 gas and R123a gas for R12 have been developed. Instead of R22 and R502, alternative fluid mixtures R404A, R407C and R507 were found.
A second hazard surrounding refrigerants is the greenhouse effect in the atmosphere. For this effect, called the Global Warming Factor (GWP), CO2 gas is taken as a reference.
The ozone layer and the coolant substances that have an effect on the natural environment can be classified under the following main headings:
1-Bromofluorocarbons or Halons (BFC)
2-Chlorofluorocarbons (CFC)
3-Hydrochlorofluorocarbons (HCFC)
4-Hydrofluorocarbons (HFC)
1-Bromofluorocarbons (Halons):
They are compounds formed by carbon, fluorine, bromine or chlordane. Halon 1301 (R13B1) can be given as an example of the substances in this group. Halons are the substances with the greatest contribution to ozone destruction.
2-Chlorofluorocarbons: (CFC)
It is composed of chlorine, fluorine and carbon. Contributing to the destruction of ozone is the coolant that is the most after the halons. Examples are R11 and R12.
3-Hydrochlorofluorocarbons: (HCFC)
They are compounds containing chlorine, fluorine, hydrogen and carbon. Ozone destruction is low but has a very high greenhouse effect. Examples of the substances in this group are R22.
4-Hydrofluorocarbons: (HFC)
Hydrogen, fluorine and carbon containing compounds. No destructive effects on ozone.
Environmental influences of refrigerants

